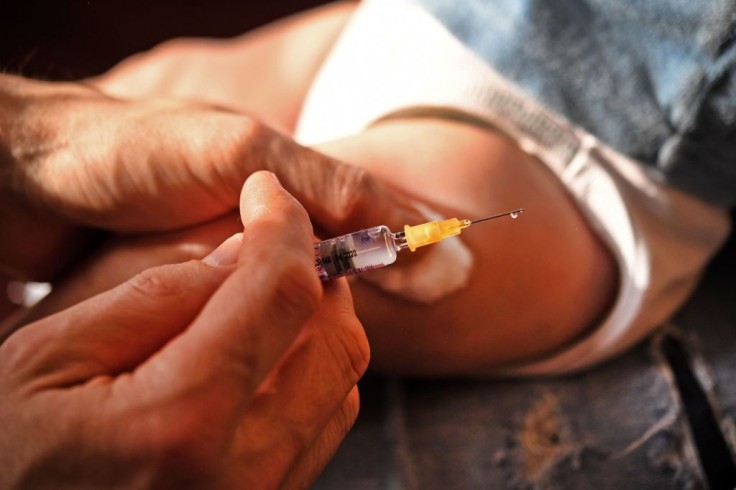
A Detroit mother has signed up her 22-month-old baby boy for Moderna's COVID-19 vaccine for kids under two years old trial. Jessica Moreno, 36, who works as a clinical pharmacist, said that, after weighing the pros and cons, she and her husband agreed to enroll their son, who is still building up his immune system, to contribute to science and to bolster their family's protection from the virus.
In an interview with Today, the mom said that she believes in the value of ethical and equitable clinical research for the COVID-19 vaccine for kids trial. She and her husband are also in agreement that the risks of being unvaccinated are greater than the side effects of the vaccines.
The mom also said that she cannot wait to tell her son when he's old enough to understand that "he got to participate in this really Earth-changing scientific breakthrough."
Moreno enrolled her son almost six months ago and didn't receive the call from the Henry Ford trial coordinator until October. Her son will be in a double-blind study for the next 14 months and, after getting the shots four weeks apart, the baby will need to undergo blood tests every few months to check for his body's response.
Not an Experiment on Children
A couple from South Carolina has also signed up their four-year-old and 14-month-old daughters to the Moderna COVID-19 vaccine for kids trial at StarMed Healthcare. The mother, a doctor, had her youngest during the pandemic and believes her baby girl will help them out of this global health crisis.
She told WCNC Charlotte that this is not an "experiment" on children because vaccine studies have been done and proven safe many times before. The parents also said that the vaccines would not get any approval if there were no proper trials for different age groups before being given to the public.
In St. Louis, Missouri, 140 children between the ages of six months to 11 years old have joined in the trials as well. About half of these kids come from non-white families, so that the experts could study the effectiveness of the Moderna vaccines based on race.
All participants have access to telemedicine and on-site check-ups while the parents keep an electronic diary to log day-to-day observations so that the experts can properly track the outcome.
Some of the parents said they were excited to be given this opportunity for their kids, especially when COVID-19 cases climbed among the younger ones. One mom said that this trial is a step closer back to normalcy for everyone in the U.S.
Strong Immune Response Seen
It comes as Moderna said that their vaccine trials for children below 11 years old but above six years old show strong immune response, effectivity, and safety. The company will soon submit the results to the U.S Food and Drug Administration (FDA) to gain emergency use authorization.
Pfizer submitted its trial's result a month earlier than Moderna, and it has been waiting for the FDA's authorization. Health officials believe that Pfizer's vaccine for younger kids will begin its rollout by November. If things go as planned, children in the U.S. could likely have options to get jabs for any of these two vaccine brands in early 2022.
Related Article: FDA Holds off Moderna Vaccine for Teens Due to Myocarditis Concerns