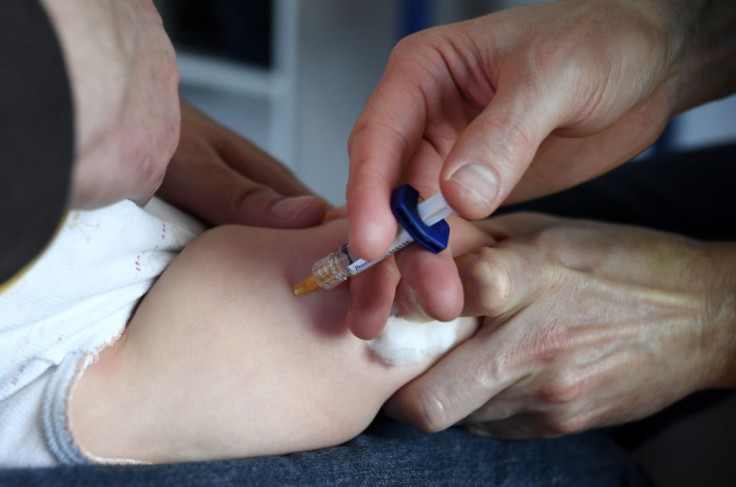
The Pfizer COVID vaccine given to children between six months to four years old failed to provide a substantial immunity response that should protect them from the virus. The pharmaceutical company said that it will be changing its plans for the ongoing clinical trials if the U.S. Food and Drug Administration (FDA) approves.
According to reports, the Pfizer COVID vaccine for this age group has been administered in two shots with three micrograms per shot. The dose is just 10 percent of the vaccine given to adults, which is at 30 micrograms per shot.
Early outcomes of the trial showed that while the shots produced the expected immunity for those under two years old, including the babies, the majority of the children between three to four years old did not produce the expected level of protection from the virus. A spokesperson for Pfizer said they plan to give a third three-microgram shot some two months after the children had their second jab.
No Safety Concerns Reported
Pfizer also confirmed that there were no safety concerns reported with the administered dosages for the youngest group. Thus, they believe that a third dose would be the right amount to deliver maximum immunity.
If the FDA will allow Pfizer to administer the third dose, the company said it will likely be able to submit data for their application for emergency use authorization (EUA) for the under five years old within the first half of 2022.
During the summer, the regulator cleared the vaccine for emergency use authorization for 12- to 17-year-old kids at 30 micrograms per shot. By the fall, the FDA also signed the approval for vaccination for kids between 5 to 11 years old at lower doses or 10 micrograms per shot.
However, these two age groups have yet to be granted full authorization because only the vaccines for adults have full approval. These age groups have also not been cleared for booster shots, which are now available for adults.
Dr. Anthony Fauci, the chief medical advisor for the White House said that while no one likes to delay the vaccines for the under five years old, especially with the Omicron COVID variant doubling cases by the day, it's imperative that the vaccine makers get it right for the safety of the children.
Pfizer's Oral Antiviral Drug
Meanwhile, Pfizer is also planning to acquire approval for its oral antiviral drug for treating COVID-19. The pill, called Paxlovid, has been shown to have an 89 percent rate of cutting the risks of death or hospitalizations among high-risk patients.
President Joe Biden said that his administration will secure 10 million Paxlovid once it has the approval of the FDA. The drug, however, cannot be used in kids but it will greatly accelerate the treatments given to COVID-19 patients. Secretary Xavier Becerra of the Health and Human Services (HHS) said that the pill is going to be a lifesaver.