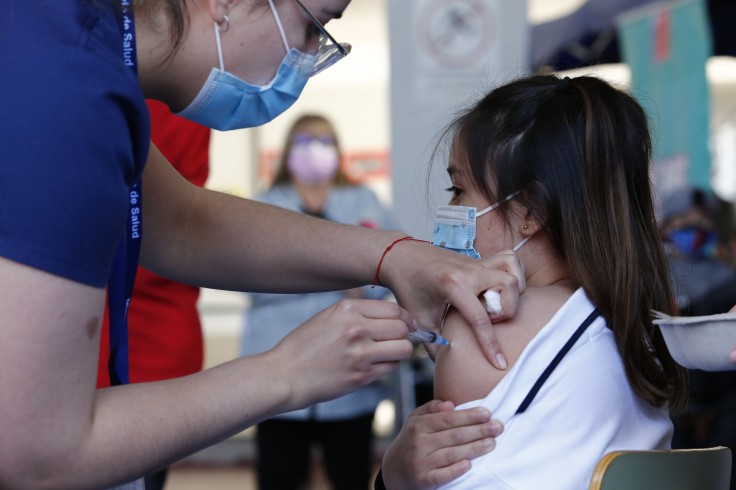
Governors across the U.S. have received word from the White House to prepare the rollout and system for COVID-19 vaccination in children between 5 to 11 years old as the U.S. Food and Drug Administration (FDA) is due to green light Pfizer's vaccine for kids.
According to a representative from the Department of Health and Human Services (HHS), the Biden administration has already bought 65 million doses of Pfizer/BioNTech vaccines meant for the below 12 years old. These supplies will be distributed across the states, including various medical organizations, as soon as the approval is greenlit.
The U.S. Centers for Disease Control and Prevention (CDC) has also started signing up providers for the COVID-19 vaccination in children. On the other hand, HHS is preparing a message campaign to encourage parents to sign up their children for the jab.
A member of the FDA committee said they would discuss Pfizer's trial results on October 26 with the pharmaceutical company and the scientists who developed the COVID-19 vaccination in children. Thus, the vaccine approval will likely come in late October or early November.
Read Also: Routine Childhood Vaccinations Dropped During the Pandemic, Causing Concerns for Parents and Doctors
Pediatricians and Family Doctors to Join in the Vaccine Rollout
The White House has also advised governors to sign up family doctors and pediatricians in private practice and pharmacies to ensure that COVID-19 vaccination in children is accessible to all. Enlisting various medical networks will help fast-track the rollout as many parents expressed the need to get their kids protected against the Delta COVID-19 variant.
Since the start of the new school year in late August, cases among children have reached 750,000 per the American Academy of Pediatrics. In the first week of October, COVID-19 cases in kids reached 24.8 percent, up from 16 percent since March 2020, the start of the pandemic.
"We have the supply," White House coronavirus response coordinator Jeff Zient confirmed. "I want to emphasize it's a different supply; the dose for kids is a different dose than adults."
Pfizer's children's vaccine consists of just a third of the dosage inoculated on adults and kids between 12 to 17. The clinical trials showed that the vaccine promoted a strong immune response against the virus and has been well-tolerated with minimal side effects by the children who volunteered during the study.
California Schools Plan Vaccine Clinics
Following the White House's message to governors, 100 schools in the Bay Area have started planning to set up vaccine clinics that will open as soon as the FDA approval is out. Gov. Gavin Newsom also said that vaccines would be required among school children following a full approval, which will be next after the emergency use approval.
Health officials in the Bay Area encouraged parents that even if their children got sick and recovered from COVID-19, vaccination will still help the T-cells mature and deliver "a long-lasting, durable response."
This will make the children's immune system more robust and likely protect them from coronavirus for the longer term, according to Dr. Monica Gandhi of the University of California San Francisco.